IBSA’s Hyaluronic Acid
What is hyaluronic acid?
From a chemical standpoint, hyaluronic acid is a polysaccharide belonging to the family of glycosaminoglycans (GAGs), composed of repeating disaccharide units of N-acetylglucosamine and D-glucuronic acid.
It has a gelatinous consistency, and its peculiar characteristics include excellent viscoelasticity and high biocompatibility1.
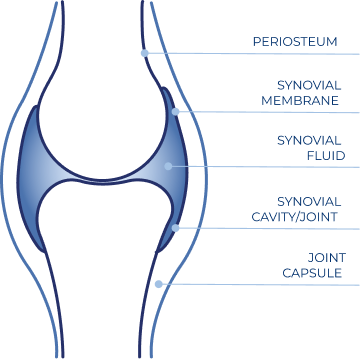
HYALURONIC ACID IN SYNOVIAL FLUID
A fundamental component of the synovial fluid and the articular cartilage matrix is hyaluronic acid (HA).
- HA is secreted into the joints by synovial cells present in the synovial membrane2
- The HA contained in the synovial fluid has a molecular weight of several millions of Daltons.


The origin of Hyaluronic Acid
Hyaluronic Acid (HA) has two main sources of origin: extraction from animal tissues and bacterial production through biofermentation.
Traditionally, hyaluronic acid was extracted from animal tissues, such as rooster combs, but this method could introduce impurities such as endotoxins and protein residues with potential immunogenic effects3.
Today, the technology of production through microbial fermentation allows the obtainment of hyaluronic acid of high purity, with low levels of proteins and endotoxins.

The transition from traditional extraction from animal sources to biofermentative production has represented a significant advancement in the production of hyaluronic acid, ensuring products of greater purity and safety, as well as better meeting the needs of modern treatments. This change highlights not only technological evolution but also a greater respect for ethical concerns related to the use of raw materials of animal origin.


ROLE OF HYALURONIC ACID IN SYNOVIAL FLUID
Hyaluronic Acid provides the viscoelastic properties to the synovial fluid (4,5)
The synovial fluid, rich in hyaluronic acid, performs crucial functions of lubrication and nutrition4 of the joint cartilage and is essential for reducing friction between the joint cartilages during movement, thus protecting the joints from wear and mechanical stress.
Synovial fluid functions
The synovial fluid is the interface between the synovial membrane and the cartilage:
- Reduces friction between joint surfaces6
- Protects the joint from mechanical stress6
With advancing age or in the case of osteoarthritis, the synovial fluid responds less to mechanical stresses because it loses its viscoelastic properties due to a reduction in the concentration and molecular weight of hyaluronic acid, and the joint is not optimally protected (1,2).
Use of
Hyaluronic
Acid
In patients suffering from osteoarthritis or other degenerative joint diseases and in case of traumas, viscosupplementation with hyaluronic acid represents a valuable therapeutic strategy for restoring joint functions, offering pain relief, and improving mobility.
In cases of arthritic disease or with advancing age, the synovial fluid inside the joints loses its viscoelastic properties due to a reduction in the concentration and molecular weight of hyaluronic acid.
Under these conditions, it becomes beneficial to restore an adequate concentration of hyaluronic acid through ultrasound-guided intra-articular injections, a treatment known as VISCOSUPPLEMENTATION.
restores the viscoelasticity of the synovial fluid5
helps reduce
pain7
improves joint functionality7


The effectiveness of infiltrative therapy depends on the characteristics of the hyaluronic acid: concentration and molecular weight.
The concentration of hyaluronic acid in healthy synovial fluid varies from 1 to 4 mg/ml9, while in the synovial fluid of joints affected by osteoarthritis it varies from 0.32 to 3.61 mg/ml1.
The higher the concentration of hyaluronic acid for infiltration, the greater the probability of recovering the properties of healthy synovial fluid9.



IBSA’s experience, thanks to the search for new production solutions and new technologies, has given rise to three generations of hyaluronic acid for infiltrative therapy:
IBSA is the first and only company to have developed, through the patented NAHYCO® Hybrid Technology, hybrid formulations of hyaluronic acid that combine high and low molecular weight hyaluronic acid, achieving one of the highest hyaluronic acid concentrations on the market (3.2%) and with rheological properties like those of endogenous synovial fluid, mimicking its activity.
The same technology has allowed the development of a new formulation with one of the highest concentrations of hyaluronic acid on the market (72 mg in 3 ml), based on combined hybrid hyaluronic acid characterized by hybrid complexes made up of high weight hyaluronic acid molecules molecular and non-sulphated sodium chondroitin, a new polysaccharide of biofermentative origin.
Currently, viscosupplementation represents one of the main conservative treatments in clinical practice for mild to moderate osteoarthritis of the knee, hip, and shoulder8 as well as in small joint, like hand OA.
It is also recommended for other pathologies of the peri-articular structures such as bursitis, capsulitis, fasciitis, enthesopathies, and tendinitis, which can occur because of trauma or from wear and tear of the joints when they are intensely stressed, for example during physical activity, in competitive sports and in some work activities that require physical effort.
LINEAR
HYALURONIC
ACID
Hybrid
HYALURONIC
ACID
COMBINED hybrid
HYALURONIC
ACID
The production process
of IBSA hyaluronic acid
Biofermentative, ultra-pure, produced in EU
IBSA’s production facilities stand out for adopting a cutting-edge production process for the manufacture of biofermentative origin hyaluronic acid (HA).
The production process of IBSA hyaluronic acid begins with the selection and isolation of a highly pure bacterial strain that is an overproducer of hyaluronic acid. This is followed by bacterial fermentation using culture media containing carbon sources (such as glucose or sucrose) and nitrogen sources (yeast extract, amino acids, and vegetable peptones) for the synthesis of biofermentative hyaluronic acid.
The process continues with two purification steps:
FIRST:
aims to remove the hyaluronic acid-producing microorganism through filtration and microfiltration
SECOND:
purification step uses ultrafiltration to remove contaminants co-precipitated with HA (such as DNA, proteins, and minerals).
Finally, the finishing and packaging of the finished product involve the removal of excess water, vacuum drying of the hyaluronic acid powder, and packaging.
This innovative and efficient production process ensures the creation of ultra-pure biofermentative hyaluronic acid of Italian origin, distinguished by the absence of extraction from animal sources and its high biocompatibility, thereby contributing to meeting the therapeutic needs of doctors and patients with high-quality and safe solutions.
The
evolution of hyaluronic acid
Hyaluronic acid (HA) represents a milestone in joint health and infiltrative therapy, thanks to its exceptional viscoelastic and biomechanical properties11.
Traditionally, HA was obtained from animal tissues through an extraction method. However, this method posed the risk of containing endotoxins and protein residues with potential immunogenic effects11.
Its evolution, driven by IBSA’s constant commitment to research and development, has led to the creation of innovative therapeutic solutions capable of significantly improving the quality of life for patients.
Thanks to the introduction of new production solutions and the development of new patented technologies, it has given rise to three generations of hyaluronic acid for infiltrative therapy.
ADVANCED PRODUCTION TECHNOLOGIES
The adoption of advanced production techniques, such as biofermentation, has allowed the development of linear formulations (FIRST GENERATION) based on ultra-pure Italian biofermentative hyaluronic acid, of high quality and safety.
THE HYBRID REVOLUTION
IBSA has developed and patented the innovative NAHYCO® Hybrid Technology which, through a controlled thermal process, has made it possible to obtain hybrid complexes of hyaluronic acid with high and low molecular weight, both of biofermentative origin (SECOND GENERATION).
With the use of the same technology, it was possible to combine high molecular weight hyaluronic acid and non-sulphated sodium chondroitin, a new polysaccharide of biofermentative origin and obtain combined hybrid complexes of hyaluronic acid (THIRD GENERATION).
Hybrid innovation has given rise to the development of hybrid formulations of very high concentration hyaluronic acid with a low viscosity allowing for easy extrusion from the syringe for greater patient comfort as well as improved efficacy and long lasting of the treatment.